In the world of pharmacology, ensuring the safety and effectiveness of medications is paramount. One critical aspect of this pursuit is understanding how drugs interact with each other when taken together. These interactions, known as Drug-Drug Interactions (DDIs), can have profound effects on a patient’s response to medication. Here, we delve into the complexities of DDIs and how they are studied.
Understaning the Potential of Drug-Drug Interaction Studies (DDIs)
Drug-Drug Interactions (DDIs) are a critical aspect of pharmaceutical research and healthcare. Understanding how different medications interact with each other can lead to safer and more effective treatments.
- Comprehensive Literature Review
Start by conducting a thorough literature review. Explore existing research on the drugs in question, paying close attention to any reported DDIs. Scientific journals, databases, and regulatory agency reports can be valuable sources of information. Understanding the potential interactions is the first step in mitigating risks.
- Consult with Experts
Engage with experts in pharmacology, toxicology, and clinical pharmacy. Their insights and expertise can help you interpret existing DDI data and design new studies if necessary. Collaborating with professionals who specialize in drug interactions is invaluable when assessing the safety of drug combinations.
- Employ Advanced Modeling and Simulation
Take advantage of advanced modeling and simulation techniques. These tools allow researchers to predict potential DDIs, even before conducting clinical trials. Through computational modeling, scientists can assess the likelihood and severity of interactions, helping to guide clinical study designs and dosage recommendations.
- Conduct In Vitro Studies
In vitro studies involve experimenting with isolated cells or tissues. For DDIs, these studies typically assess how drugs affect specific enzymes or transporters involved in drug metabolism and distribution. In vitro data can provide crucial insights into potential interactions and guide further investigations.
- Utilize Preclinical Studies
Before moving to human trials, conduct preclinical studies in animal models. These experiments can reveal how drugs interact within a living system. They help establish a foundation for understanding potential DDIs’ mechanisms and effects, enabling researchers to make informed decisions about clinical trials.
- Clinical Trials with DDIs in Mind
When designing clinical trials, keep DDIs in mind. Include a diverse patient population to account for genetic variations that may influence how individuals metabolize drugs. Monitor patients closely for unexpected interactions, and adjust treatment plans accordingly.
- Leverage Real-World Data
After a drug reaches the market, continue monitoring for DDIs using real-world data. Electronic health records, pharmacovigilance databases, and post-marketing surveillance can help identify previously unseen interactions. Timely detection allows for swift regulatory action when necessary.
- Implement Risk Mitigation Strategies
Develop risk mitigation strategies based on DDI findings. This may involve adjusting dosages, revising treatment guidelines, or issuing warnings to healthcare providers and patients. The goal is to maximize the benefits of drug combinations while minimizing potential harm.
- Regulatory Compliance
Adhere to regulatory guidelines and requirements. Regulatory agencies, such as the FDA, mandate rigorous DDI assessments as part of the drug approval process. Ensuring compliance is essential for market approval and patient safety.
- Continuous Learning and Improvement
The field of DDIs is continually evolving. Stay updated on the latest research, methodologies, and regulatory changes. Learning from past experiences and embracing innovation is key to making full use of DDIs in drug development and healthcare.
Conclusion
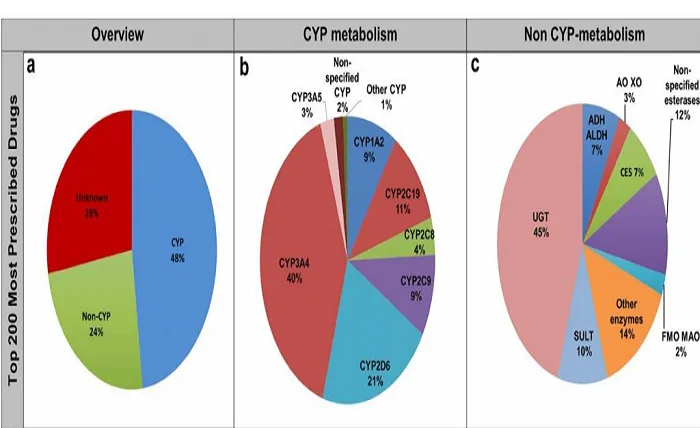
The study of DDIs primarily takes place in vitro, using well-defined enzymatic reactions as benchmarks. Researchers aim to uncover potential mechanisms of DDIs and obtain kinetic parameters for further analysis. This involves understanding how drugs affect the metabolic behavior of co-administered drugs.